In the realm of chemistry, the fascination with compounds and their behaviors often raises intriguing questions, one of which is whether compounds can be separated by physical means. At the heart of this inquiry is a fundamental understanding of what compounds are and how they interact with their environment.
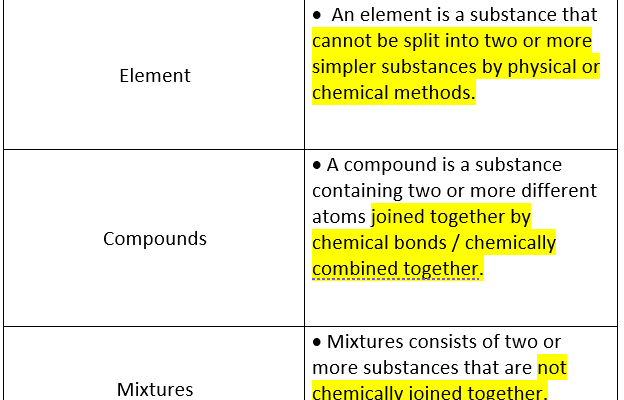
A compound is a substance formed when two or more chemical elements are chemically bonded together. The nature of these bonds—ionic, covalent, or metallic—determines the characteristics of the compound. Unlike mixtures, where the components retain their individual properties and can often be separated by physical means, compounds are characterized by a fixed composition and distinct properties that can be vastly different from the constituent elements.
When considering the separation of compounds, its important to recognize that their chemical bonds are not easily undone. Physical means—such as filtration, evaporation, or decantation—are typically employed in the separation of mixtures. These methods exploit differences in physical properties, such as particle size, state of matter, or volatility, to separate substances without altering their chemical identities.
However, in the case of compounds, the bonds that hold the elements together must usually be broken to separate the components, a process that is chemical rather than physical. For example, separating water (H2O) into hydrogen and oxygen requires electrolysis, a chemical process that involves the application of an electric current.
Despite the typical necessity for chemical processes, there are fascinating exceptions where physical means can aid in the separation of compounds. Some complex compounds can undergo physical changes that facilitate separation, often through advanced techniques such as sublimation or crystallization in controlled environments.
Furthermore, recent advancements in nanotechnology and material sciences have opened new avenues for exploring the separation of compounds. Techniques involving selective adsorption and the use of membranes have shown promise in separating components of certain compounds without breaking chemical bonds.
The quest to separate compounds by physical means is not just an academic exercise; it has practical implications in fields ranging from pharmaceuticals to environmental science. As researchers continue to push the boundaries of what is possible, the line between chemical and physical separation may blur, leading to innovative solutions for complex scientific challenges.
In conclusion, while traditional methods of separating compounds typically involve chemical processes, ongoing research and technological advancements suggest that physical means could play a more significant role in the future. The science of separation is as dynamic and evolving as the compounds it seeks to unravel, promising exciting developments in the years to come.
body {
font-family: Arial, sans-serif;
margin: 0;
padding: 0;
line-height: 1.6;
}
.content {
padding: 20px;
max-width: 800px;
margin: auto;
}
h1, h2 {
color: #333;
}
p {
color: #555;
}
.highlight {
background-color: #f0f0f0;
padding: 5px;
}
In the realm of chemistry, the fascination with compounds and their behaviors often raises intriguing questions, one of which is whether compounds can be separated by physical means. At the heart of this inquiry is a fundamental understanding of what compounds are and how they interact with their environment.
Understanding Compounds
A compound is a substance formed when two or more chemical elements are chemically bonded together. The nature of these bonds—ionic, covalent, or metallic—determines the characteristics of the compound. Unlike mixtures, where the components retain their individual properties and can often be separated by physical means, compounds are characterized by a fixed composition and distinct properties that can be vastly different from the constituent elements.
The Challenge of Separation
When considering the separation of compounds, its important to recognize that their chemical bonds are not easily undone. Physical means—such as filtration, evaporation, or decantation—are typically employed in the separation of mixtures. These methods exploit differences in physical properties, such as particle size, state of matter, or volatility, to separate substances without altering their chemical identities.
However, in the case of compounds, the bonds that hold the elements together must usually be broken to separate the components, a process that is chemical rather than physical. For example, separating water (H2O) into hydrogen and oxygen requires electrolysis, a chemical process that involves the application of an electric current.
Exceptions and Explorations
Despite the typical necessity for chemical processes, there are fascinating exceptions where physical means can aid in the separation of compounds. Some complex compounds can undergo physical changes that facilitate separation, often through advanced techniques such as sublimation or crystallization in controlled environments.
Furthermore, recent advancements in nanotechnology and material sciences have opened new avenues for exploring the separation of compounds. Techniques involving selective adsorption and the use of membranes have shown promise in separating components of certain compounds without breaking chemical bonds.
The Future of Compound Separation
The quest to separate compounds by physical means is not just an academic exercise; it has practical implications in fields ranging from pharmaceuticals to environmental science. As researchers continue to push the boundaries of what is possible, the line between chemical and physical separation may blur, leading to innovative solutions for complex scientific challenges.
In conclusion, while traditional methods of separating compounds typically involve chemical processes, ongoing research and technological advancements suggest that physical means could play a more significant role in the future. The science of separation is as dynamic and evolving as the compounds it seeks to unravel, promising exciting developments in the years to come.
Sparkling Discoveries on the Horizon
As we peer into the future of chemistry, the potential for innovative breakthroughs is tantalizing. Imagine materials that can selectively capture and release specific elements under certain conditions, or compounds that can be gently coaxed apart using light, sound, or magnetic fields. These are not just flights of fancy but active areas of research driven by a deep desire to understand and manipulate the world at a molecular level.
The implications of mastering such techniques are profound. In the pharmaceutical industry, for example, being able to separate compounds with precision could lead to purer, more effective medications. In environmental science, it might allow us to efficiently extract valuable materials from waste streams without harmful byproducts.
Ultimately, the journey to separate compounds by physical means is a testament to human ingenuity and the relentless pursuit of knowledge. As our understanding of chemical interactions deepens, who knows what sparkling discoveries await just beyond the horizon?
I found the discussion on how physical means can sometimes aid in separating compounds quite intriguing. It’s fascinating to see how advancements in technology are pushing the boundaries of traditional chemistry.
I appreciate the detailed explanation on chemical bonds and why they require chemical processes to break. This foundational knowledge is crucial for anyone studying chemistry.
This article provides a clear and concise explanation of the difference between compounds and mixtures, which is often a point of confusion for many. The examples given, like water electrolysis, are very helpful in understanding the concept.
The article effectively highlights the importance of understanding chemical bonds when discussing compound separation. It’s both informative and engaging, making it a valuable resource for learners.
The article does an excellent job at explaining why compounds cannot be separated by physical means, which is a common misconception. The clarity with which this complex topic is presented is commendable.
Great read! I especially liked the mention of nanotechnology and material sciences as emerging fields that could change our approach to compound separation. Very forward-thinking!